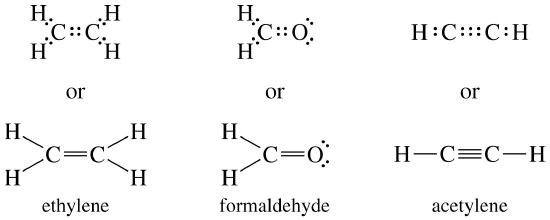
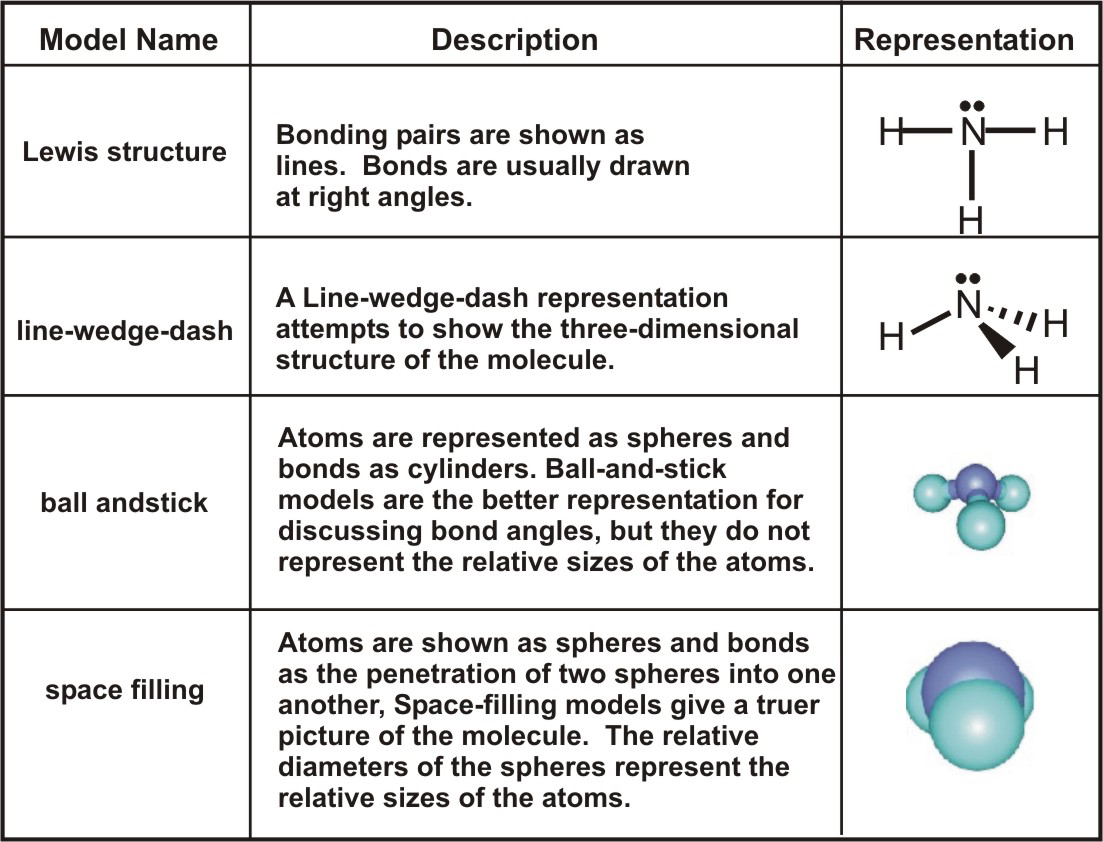
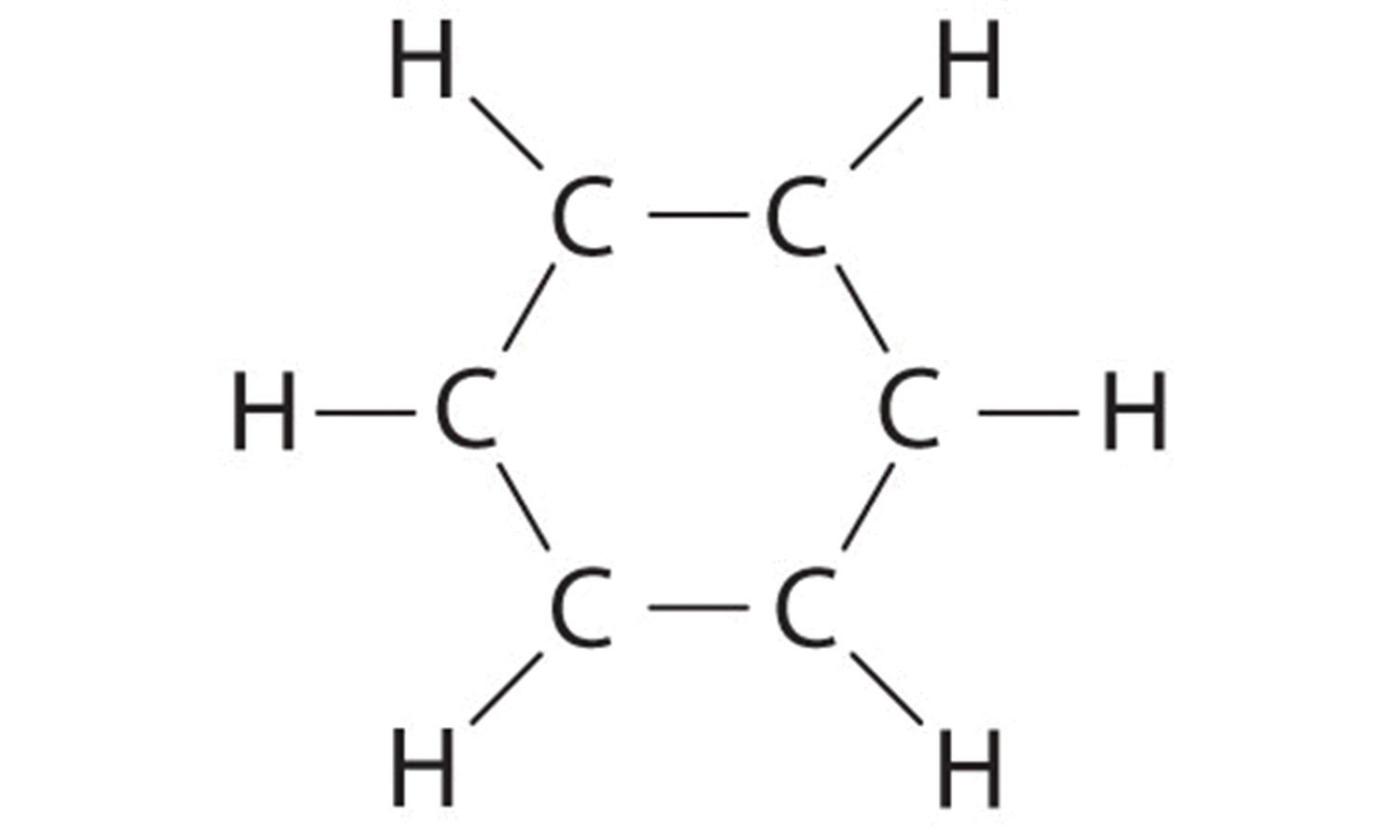
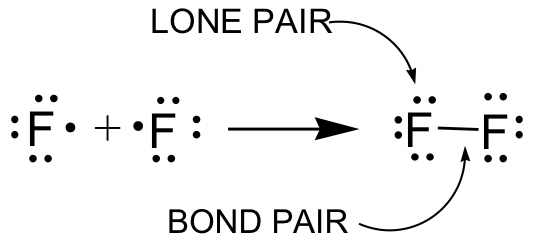
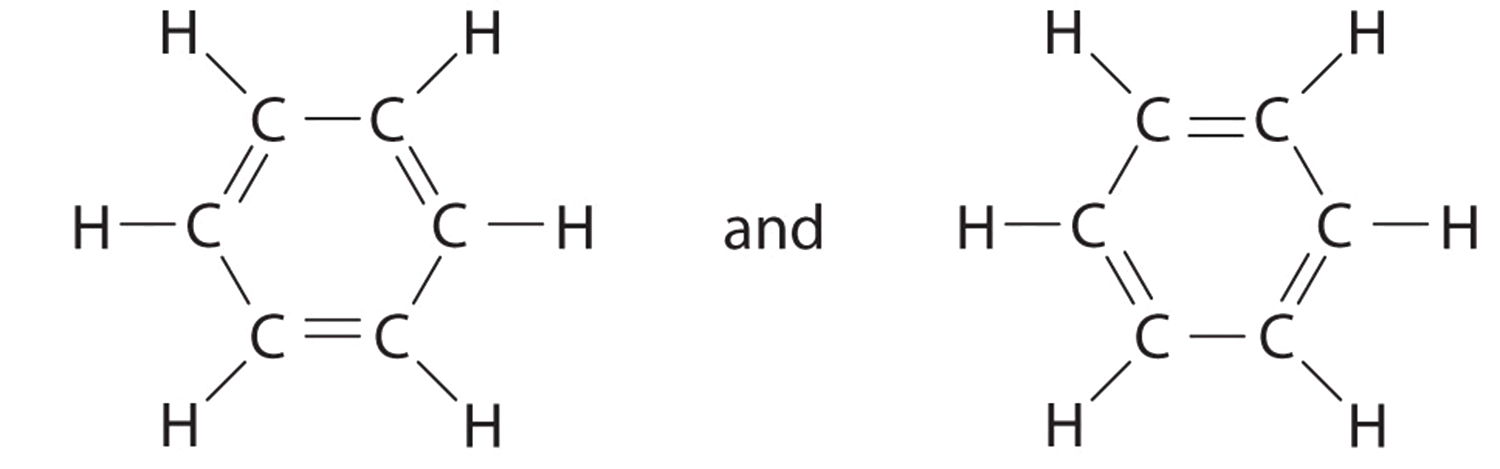Lewis structure of NaCl - How to draw Lewis structure of NaCl, Polar or Non-polar molecule, and Crystal Structure with FAQs

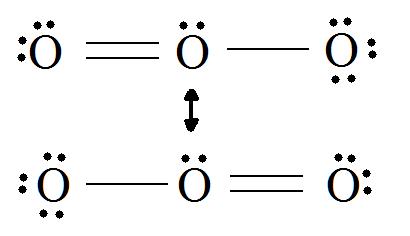
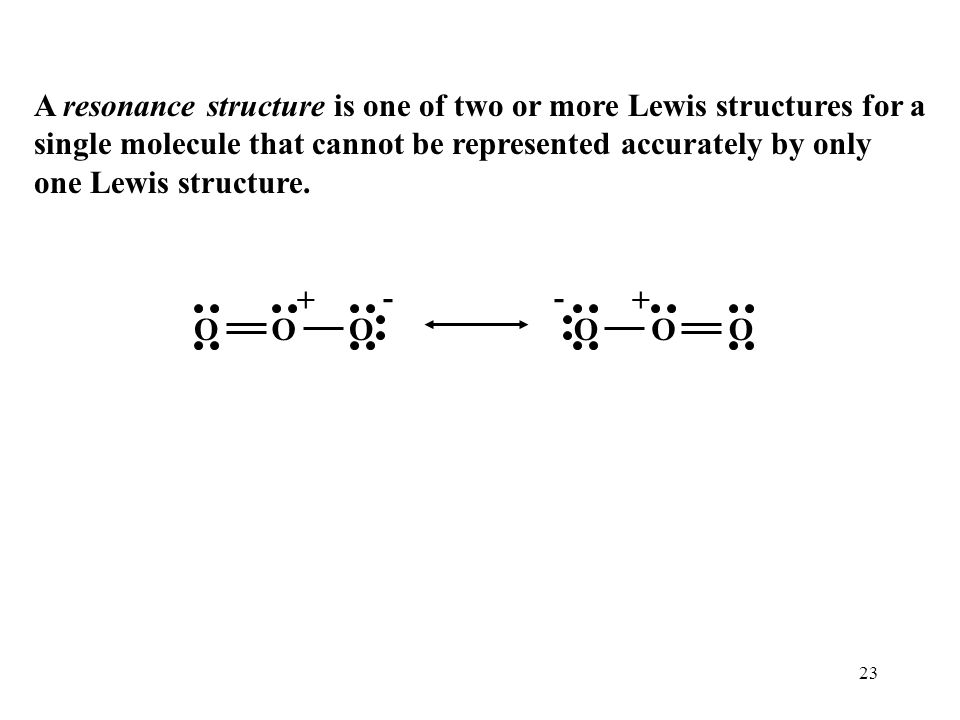
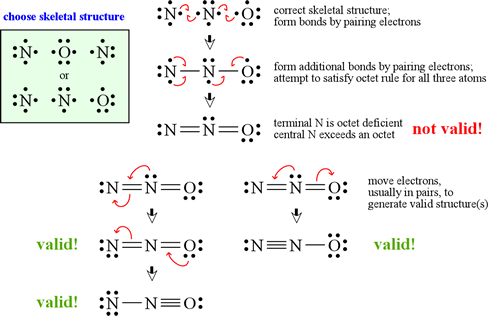
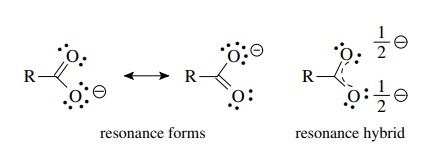
C HEM 167 F INAL R EVIEW Part 2. R ESONANCE S TRUCTURES Compound that cannot be represented by only one Lewis structure. Determine resonance structures: - ppt download

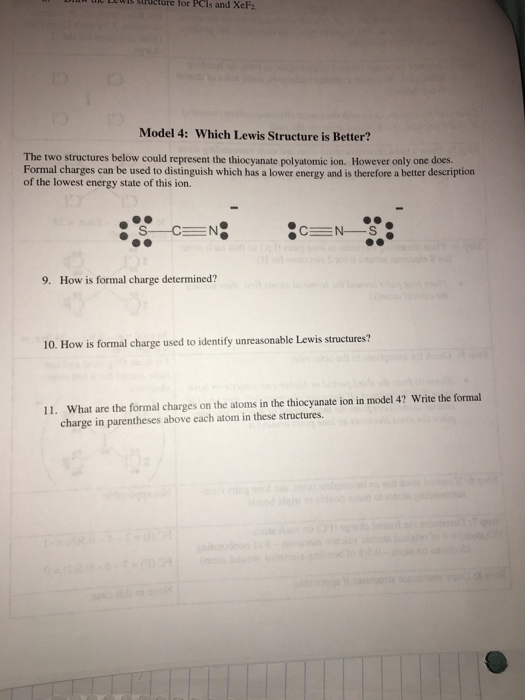
Explain why `CO_(3)^(2-)` ion cannot be represented by a single Lewis structure. How can it be best represented? - Sarthaks eConnect | Largest Online Education Community
![What is the total number of electrons in the correct Lewis dot formula of the sulfite ion?(A) $26$(B) $32$(C) $30$(D) \\[8\\] What is the total number of electrons in the correct Lewis dot formula of the sulfite ion?(A) $26$(B) $32$(C) $30$(D) \\[8\\]](https://www.vedantu.com/question-sets/fc3a63b8-5241-4c08-8ebd-4f609dc5fb094773754716586340753.png)